BEYOND EPILEPSY SYMPTOMS: UNVEILING DUAL DIAGNOSIS WITH WHOLE EXOME SEQUENCING
Epilepsy is a neurological disorder1 that is often complex to diagnose and to treat. For many patients, epilepsy has a genetic etiology. As there are hundreds of genes associated with epilepsy pinpointing the associated gene is not always straightforward. Further complicating this clinical picture is new research showing that between 2% to 7% of patients with epilepsy, or other genetic disorders, have more than one genetic condition. Traditional genetic testing approaches, which are driven by specific indications, often fail to identify all etiologies for these patients. Whole Exome Sequencing (WES) has emerged as a transformative tool in this context. WES targets the protein-coding regions within genes and can pinpoint the etiology of genetic conditions like epilepsy. This ability to detect multiple genetic conditions in one sweep can end the often frustrating and lengthy diagnostic odyssey that some patients endure. By employing WES early in the diagnostic process, the chance of missing a diagnosis can be significantly reduced, thus helping ensure patients receive accurate and timely treatments that are tailored to them.
IDENTIFYING GENETIC VARIANTS IN EPILEPSY
Epilepsy is classified as a disorder that has genetic heterogeneity, meaning that it can be caused by variants within a multitude of genes. By using comprehensive genetic tests like WES, specific genetic variants that contribute to epilepsy can be identified for clinical review. These variants may be found in genes responsible for ion channel function, neuronal development, synaptic transmission, among others.
GENETIC SYNDROMES
Epilepsy can be an isolated finding for many patients. However, there are several syndromes that have epilepsy as a clinical feature too.
Dravet Syndrome is a severe form of epilepsy, typically manifesting in the first year of life2 and characterized by frequent, prolonged seizures, and developmental delays and regression. This syndrome is caused by variants in the SCN1A gene which is pivotal for proper electrical signaling in the brain. Dravet Syndrome is a lifelong condition, managing which often requires a multidisciplinary approach encompassing antiepileptic drugs, dietary therapies, and sometimes surgical interventions.
Another syndrome, Rett Syndrome, usually has an onset between 12-18 months of age and leads to progressive loss of developmental milestones, motor skills and speech3. Caused by mutations in the MECP2 gene, many patients with this syndrome experience seizures. Although no cure exists, therapeutic interventions focus on managing symptoms and improving the quality of life.
Both syndromes exemplify the critical role of genetics in elucidating the pathophysiology of complex neurological disorders, paving the way toward personalized medicine and advanced therapeutic strategies.
EPILEPSY AND DUAL DIAGNOSIS
Sometimes, epilepsy is not an isolated condition; it can occur alongside other genetic conditions. These patients have a “dual diagnosis,” which new data shows that 2% to 7% of patients have. For these patients, epilepsy might be present along with cardiac, metabolic, or other symptoms. The combination of these symptoms can make identifying their cause difficult. Understanding the genetic etiology of these dual diagnoses is important for clinicians, personalized medicine, tailoring treatments, and interventions to the individual’s specific needs.
THE POWER OF WHOLE EXOME SEQUENCING
WES stands as a powerful, transformative tool in the realm of genetics and genomics4. In the past, the clinical approach for making a diagnosis in a patient involved testing for specific genes or on panels of related genes. While this can help some patients and their families get the diagnosis they need, for many these targeted approaches do not provide the answers needed. For numerous clinicians, patients, and their families, obtaining an accurate diagnosis can be a long, arduous journey, often termed as the “diagnostic odyssey”.
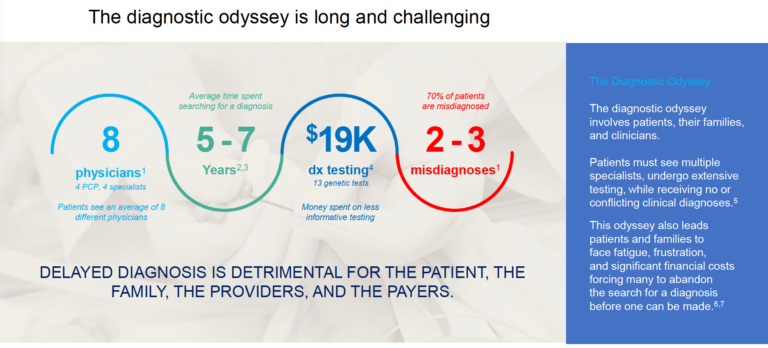
WES has significantly altered this scenario, pinpointing the exact genetic mutations that lead to a disorder is now much easier, enabling diagnoses to be made that inform management strategies. Due to the comprehensiveness of WES, the diagnostic yield of this testing is higher for many indications compared to more targeted testing.
WES can empower patients, clinicians, and researchers with a powerful tool that can show the depth of genetic information, driving innovative breakthroughs in diagnostics and therapeutics. Consequently, WES not only underscores our current genetic and genomic exploration, but also paves the way towards a future where patient care and management is able to be performed more rapidly, accurately, and comprehensively than ever before.
WES AND DUAL DIAGNOSIS FOR CLINICIANS
When clinicians have patients with complex symptoms and phenotypes leading to many different health concerns, genetic testing panels can be ordered for the patient where the genes assessed could identify a variant associated with a particular disease. While these panels may be able to identify one causative condition in these patients, panel testing might miss other conditions that they have. WES can often be the most straightforward way to make a dual diagnosis for patients with multiple etiologies for their symptoms and phenotype.
CASE STUDY: UNCOVERING DUAL DIAGNOSES
To illustrate the power of WES and its utility, let us explore a case study where WES has uncovered dual diagnoses in individuals with epilepsy.
- Epilepsy and Ventricular Arrhythmias: A patient with a history of seizures and developmental delay was seen in the ICU due to ventricular tachycardia and cardiac arrest. While the patient was suspected to have TANGO2 deficiency disorder (associated primarily with intellectual and developmental delays, seizures, and acute metabolic crises), other conditions could not be ruled out. On WES, the patient was identified as having a variant within the TANGO2 and KCNQ1 genes. KCNQ1 is associated with Long QT syndrome type 1, an arrhythmia disorder associated with cardiac arrest during periods of exercise and heightened emotions. While both conditions are associated with arrhythmias, the cause of arrhythmias differs for each condition. Here, WES allowed for an additional diagnosis to be made that this patient’s clinicians can use to help manage their care.
NEW RESEARCH ON DUAL DIAGNOSIS
Recently, there has been exciting new data presented that shows exome sequencing effectiveness, such as in WES, with identifying a dual diagnosis. At the 2023 National Society of Genetic Counselors (NSGC) 42nd Annual Meeting and the 2023 American Society of Human Genetics (ASHG) Annual Meeting, researchers from Baylor Genetics showed that nearly 2-7% of all patients with a genetic disorder that underwent WES testing were shown to have a dual diagnosis.
Significant findings like this show the power of using WES versus single-gene testing or panels. Information gleaned from WES can inform clinicians and allow for dual diagnoses to be made for their patients.
BAYLOR GENETICS’ EXPERTISE
A genetics laboratory, like Baylor Genetics, can play a crucial role in the genetic testing process. These labs specialize in the analysis and interpretation of genetic data generated through WES and other types of testing.
Baylor Genetics’ ability in interpreting complex genetic data is instrumental in finding the genetic basis of epilepsy and helping to make dual diagnoses. For 40-years, Baylor Genetics has engaged in a multidisciplinary approach that combines bioinformatics, clinical genetics, and molecular biology to provide clinicians with actionable insights into their patients’ conditions. We couple the fastest and most comprehensive precision diagnostics options with the support of genetic counselors to help clinicians and patients end the diagnostic odyssey and guide medical management.
THE BAYLOR GENETICS TEST MENU
Baylor Genetics has been at the forefront of using advanced sequencing techniques like WES and whole genome sequencing to unravel the genetic complexities of epilepsy and making dual diagnoses. Our comprehensive test menu encompasses a wide array of genetic tests, making it a valuable resource for clinicians, genetic counselors, and patients seeking answers.
CONCLUSION
Epilepsy is a complex neurological disorder caused by variants in many genes. In many cases, patients with epilepsy often have variants in other genes that can lead to additional symptoms. Baylor Genetics, with its extensive test menu and WES capabilities, is at the forefront of genetic testing. Our WES offers a rapid, comprehensive approach to allow clinicians to make a diagnosis, whether for epilepsy on its own or as part of a dual diagnosis.
By further exploring new science with dual diagnoses, researchers and clinicians can better understand the underlying cause of a patient’s symptoms and develop effective targeted treatments for them. As genetic testing and research continue to advance, there is hope for improved outcomes and a higher quality of life for individuals living with epilepsy and other conditions.
Click here to connect with a member of our team for more information on how to order WES: sales@baylorgenetics.com
References:
- https://medlineplus.gov/ency/article/000694.htm
- https://www.ninds.nih.gov/health-information/disorders/dravet-syndrome
- https://www.mayoclinic.org/diseases-conditions/rett-syndrome/symptoms-causes/syc-20377227
- https://www.sciencedirect.com/topics/nursing-and-health-professions/whole-exome-sequencing
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5739118/