The Economic Burden of Rare Diseases: Impacts & Challenges
While rare diseases are individually rare, collectively they are quite common. According to the National Organization for Rare Disorders, approximately 10% of all people worldwide have at least one rare disease.1 These diseases can pose a significant burden on individuals, their families, healthcare systems, and societies. The economic impact of rare diseases includes direct medical costs as well as other indirect expenses.
How Common are Rare Diseases?
Currently, there is no single accepted definition for rare disease:1,2
- In the United States, a rare disease is characterized by the Food and Drug Administration as a condition affecting fewer than 200,000 This is approximately 1 in 1,700 people.
- In Europe, a rare disease is defined as one affecting fewer than 1 in 2,000
- In Japan, a rare disease is defined as one affecting fewer than 1 in 2,500
While some rare diseases affect hundreds or even thousands of people, others are so rare that there may only be a handful of people affected worldwide.1,2
As of today, about 7,000 rare diseases have been identified worldwide and many do not have a known cause.3 Although a complete understanding of the causes of all rare diseases is still a work in progress, scientists continue to discover more about these diseases over time. Diverse factors can cause rare diseases and can include any of the following:
- Genetic etiologies, which can be inherited from a parent or occur just in that individual
- Infectious diseases
- Birth defects
- Rare cancers
- Autoimmune diseases
- Environmental factors
Economic Challenges Faced by Healthcare Systems, Patients, & Families
In a study conducted by Yang et al. in the Orphanet Journal of Rare Diseases, a prevalence-based approach was utilized to assess the substantial national economic burden associated with rare diseases. Research shows an estimated 379 rare diseases collectively affect approximately 15.5 million children and adults in the US. These diseases resulted in a total economic burden of $997 billion.4
The $997 billion included:4
- Direct medical costs of $449 billion (45%)
- Indirect costs of $437 billion (44%)
- Non-medical costs of $73 billion (7%)
- Healthcare costs not covered by insurance of $38 billion (4%)
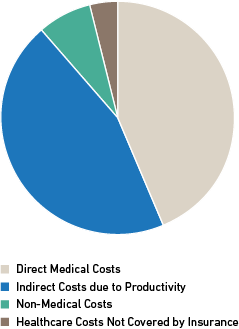
Figure 1: Economic Challenges Faced by Healthcare Systems, Patients & Families 4
Medical cost drivers for rare diseases include hospital inpatient care (32%) and prescription medication (18%), plus indirect such as labor market productivity losses.4
Direct Medical Costs
Rare diseases often require specialized medical care, including diagnostic tests, treatments, and medications. Associated costs not only affect individuals and families but also place a strain on healthcare systems and have broader societal implications.
“Patients that have chronic or recurring conditions often require expensive medications, and specialized or frequent care. Healthcare systems are doing the best they can to support these patients as well as their other patients,” said Robert Rigobello, CGC, Manager of Medical Affairs at Baylor Genetics. “However, these systems only have so much bandwidth to provide care. Especially in post-COVID times, many are struggling to keep up with the demand for services.”
The rarity and complexity of these diseases make it difficult to develop cost-effective treatments, resulting in higher drug prices. Moreover, the need for multiple healthcare professionals, extensive testing, and hospitalizations further adds to the financial burden.
Indirect Medical Costs
Indirect medical costs refer to the financial expenses incurred because of a medical condition or treatment that are not directly related to the actual medical procedures or services. These costs include patient and caregiver productivity loss, reduced work hours, career disruptions, and even leaving jobs altogether to provide care for patients with complex medical needs.
Non-medical Costs
The economic burden of rare diseases extends beyond medical costs, encompassing significant non-medical expenses. Many rare diseases require home modifications to accommodate the needs of patients. These modifications can include wheelchair accessibility, installing ramps, handrails, or specialized equipment. The cost of these modifications can be substantial, adding to the economic burden.7
Addressing these non-medical costs is essential to alleviate the financial strain on individuals and families affected by rare diseases and to improve their overall quality of life. Implementing financial assistance programs, improving access to support services, and promoting flexible work arrangements can offer relief and support to those facing these challenges.
Challenges
Several challenges that contribute to the economic burden of rare diseases include:
- Diagnostic Barriers: These refer to challenges that hinder the process of accurately identifying a medical condition leading to diagnostic odysseys. These barriers can contribute to both direct and indirect medical costs associated with rare diseases. Diagnostic barriers often lead to a prolonged and inefficient diagnostic process, which can result in multiple healthcare consultations, referrals to different specialists, and a series of tests and procedures.5
- Treatment Barriers: These barriers are obstacles that impede access to appropriate and timely medical care for individuals with a specific condition. Although the Affordable Care Act has expanded health insurance coverage, individuals with rare diseases continue to face challenges in accessing essential healthcare. Even when treatments are accessible, they are often expensive, with the cost of orphan drugs for rare diseases reaching tens or hundreds of thousands of dollars per patient per year.
Additionally, the utilization of off-label prescriptions not sanctioned by the FDA presents another notable concern. This often leads to off-label prescribing, which is common in the US, as one in five prescriptions are written for off-label use. With limited FDA-approved treatments available for rare diseases, off-label prescriptions have become a vital option for many patients.6 - Research and Development Barriers: Rare diseases often receive limited research funding and attention compared to more prevalent conditions. Conducting research on rare diseases can be difficult due to limited patient populations and greater incentives for studying these conditions over more potentially profitable ones. This makes it challenging to gather sufficient evidence for developing new treatments, diagnostics, and interventions, further impeding progress in the field. This further perpetuates the economic burden by limiting access to affordable and innovative therapies.
- Patient and Caregiver Productivity Barriers: Rare diseases can result in functional limitations and disabilities, reducing patients’ ability to participate in the workforce. The time and effort required for caregiving duties, frequent medical appointments, and managing complex treatments leave little room for pursuing a career or maintaining a stable income. As a result, individuals and their families experience reduced earning potential and financial stability.
- Geographic Barriers: In the case of many rare diseases, there are only a handful of specialists who have expertise with a rare condition. As a result, patients often face burdensome challenges when traveling long distances to reach their healthcare providers.6 These barriers can result in delays or missed opportunities for diagnosis, leading to potential gaps in care. Various factors such as financial constraints, lack of transportation, fear or anxiety associated with medical procedures, language barriers, or limited health literacy often contribute to the above barriers.
“Many times, there aren’t enough centers or nearby centers that offer care for many of these patients. There may be a primary healthcare center trying to manage care, especially early on into a patient’s diagnostic odyssey,” said Rigobello. “That said, a primary healthcare provider taking care of these patients might not be familiar or have experience with a specific rare disease. They do the best they can, but many of these patients really need a multidisciplinary team to comprehensively support them.”
Addressing these patient-related barriers requires tailored strategies such as patient education, improved communication, financial assistance programs, and support services to facilitate and encourage patients to actively engage in the diagnostic process and follow through with necessary referrals and appointments.
Insurance Coverage
Recent efforts have been made to enhance insurance coverage for genetic testing services, acknowledging the transformative potential of advancements in genetic testing, especially genome sequencing, for rare disease diagnoses. The incorporation of Rapid Whole Genome Sequencing (rWGS) into state Medicaid programs, such as Michigan Medicaid and California Medi-Cal, marks a noteworthy advancement in reducing the diagnostic hurdles faced by individuals with rare diseases. This initiative also serves to enhance the availability of advanced testing methods, making them more attainable for a broader population.
Moreover, the field of reimbursement opportunities for rWGS continues to emerge. Cigna’s medical policy on Whole Genome Sequencing (WGS) coverage, including both inpatient and outpatient WGS as well as inpatient rWGS, is adopting guidelines that affirm the medical necessity of these tests, particularly for critically ill children in intensive care units whose underlying conditions are yet to be identified. In addition to this, insurance carriers like UnitedHealthcare have widened their coverage to encompass exome and genome sequencing, presenting enhanced possibilities for patients to explore genetic testing options.
By improving insurance coverage for genetic testing and expanding state Medicaid programs to include coverage for WGS services for specific individuals, the burden of diagnosing rare diseases can be alleviated. However, a substantial path lies ahead in fully realizing the potential of these advancements and ensuring equitable access for all.
Alleviating the Burden
To harness the benefits of genomics, it is necessary to expedite the implementation of genomic medicine in clinical practice. It is estimated that there is currently a 17-year lag with implementing research into the clinic.8 Next-generation sequencing technologies, specifically genome and exome sequencing, offer a promising solution to help reduce the global burden of the diagnostic odyssey associated with genetic and rare diseases.
Per the American College of Medical Genetics and Genomics, exome and genome sequencing are recommended as first- or second-tier tests for pediatric patients with congenital anomalies, developmental delays, or intellectual disabilities.9 Early and accurate diagnoses through these tests empower patients with valuable knowledge about their conditions, facilitating better disease management, reduction in medical costs, and improving their overall quality of life.
For over 40 years, Baylor Genetics has been committed to advancing genetic testing and providing diagnostic solutions to healthcare providers and their patients. Baylor Genetics offers Whole Exome Sequencing (WES) and WGS as part of its commitment to improving patient outcomes and reducing the diagnostic odyssey. Turnaround time ensures a final report in as little as 5 days, promoting timely decision-making and enhanced patient management for healthcare providers and their patients.
Furthermore, Baylor Genetics is also actively involved in research initiatives for rare diseases, including the Undiagnosed Diseases Network (UDN) and the Medical Genome Initiative (MGI).
The UDN, funded by the National Institutes of Health Common Fund, was established to address the need to more effectively diagnose rare conditions. As the exclusive sequencing core for the UDN’s exome and genome sequencing, Baylor Genetics plays a crucial role in providing much needed diagnoses to patients and their families.
In a study involving researchers from Baylor Genetics and authored by Kimberly Splinter, Director, UDN Coordinating Center at Harvard Medical School, the article titled, Effect of Genetic Diagnosis on Patients with Previously Undiagnosed Disease, revealed that among the patients included, the average cost of care before being accepted into the UDN was substantial. Among the patients who received a diagnosis through the UDN, the average cost of care before acceptance was $305,428. Following the UDN’s evaluation, the average cost decreased to $18,903, resulting in a savings of approximately 94% compared to the original cost. These findings align with recent cost-effectiveness analyses for genome sequencing and emphasize the potential of the UDN’s approach in reducing the financial burden associated with a lengthy and expensive diagnostic journey.10
In addition to the UDN, Baylor Genetics supports MGI. This consortium, founded by leading institutions with extensive experience in genome research, seeks to understand the genetic basis of diseases. The MGI advocates for early utilization of clinical WGS as a powerful tool to provide precise molecular diagnoses, leading to more effective medical management. This approach has the potential to decrease the number of unresolved, complex, costly, and chronic genetic disease cases, especially among newborns and children.11
Together, We Can Make a Difference
The economic challenges posed by rare diseases have far-reaching impacts, affecting individuals, families, healthcare systems, and societies. Recognizing the scale of the economic burden of rare diseases is the first step toward finding sustainable solutions. To comprehensively assess the burden of rare diseases, systematic studies should encompass multiple dimensions of healthcare costs.
Addressing this burden requires a comprehensive approach that involves:
- Increased research funding
- Improved access to specialized care
- Financial assistance programs
- Support for patients and caregivers
Additionally, investing in diagnostic tools, newborn screening, and the development of new therapies for rare diseases is crucial.4
By fostering a diverse, equitable, and inclusive rare disease community, backed by initiatives like the UDN and MGI, Baylor Genetics works to ensure equal opportunities, representation, and prompt diagnoses for all individuals impacted by rare diseases. Together, we can drive change, create progress, and strengthen the rare disease community.
References
- Updated Rare Disease Facts and Figures from NORD – Rare & Undiagnosed Network (rareundiagnosed.org)
- Shafie, A.A., Chaiyakunapruk, N., Supian, A. et al. State of rare disease management in Southeast Asia. Orphanet J Rare Dis 11, 107 (2016). https://doi.org/10.1186/s13023-016-0460-9
- About – Genetic and Rare Diseases Information Center (nih.gov)
- Yang G, Cintina I, Pariser A, Oehrlein E, Sullivan J, Kennedy A. The national economic burden of rare disease in the United States in 2019. Orphanet J Rare Dis. 2022 Apr 12;17(1):163. doi: 10.1186/s13023-022-02299-5. PMID: 35414039; PMCID: PMC9004040.
- Diagnostics barriers and innovations in rural areas: insights from junior medical doctors on the frontlines of rural care in Peru | BMC Health Services Research | Full Text (biomedcentral.com)
- Barriers to Rare Disease Diagnosis, Care, and Treatment in the US NRD-2088-Barriers-30-Yr-Survey-Report_FNL-2.pdf (rarediseases.org)
- Rare Diseases: Although Limited, Available Evidence Suggests Medical and Other Costs Can Be Substantial, U.S. Government Accountability Office, October 2021
- Stark Z, Dolman L, Manolio TA, Ozenberger B, Hill SL, Caulfied MJ, Levy Y, Glazer D, Wilson J, Lawler M, Boughtwood T, Braithwaite J, Goodhand P, Birney E, North KN. Integrating Genomics into Healthcare: A Global Responsibility. Am J Hum Genet. 2019 Jan 3;104(1):13-20. doi: 10.1016/j.ajhg.2018.11.014. PMID: 30609404; PMCID: PMC6323624.
- Manickam K, et al. Exome and genome sequencing for pediatric patients with congenital anomalies or intellectual disability: an evidence-based clinical guideline of the American College of Medical Genetics and Genomics (ACMG). Genet Med 2021; 23:2029–2037.
- Splinter K, Adams DR, Bacino CA, et al. Effect of Genetic Diagnosis on Patients with Previously Undiagnosed Disease. N Engl J Med. 2018;379(22):2131-2139. doi:10.1056/NEJMoa1714458
- Medical Genome Initiative (medgenomeinitiative.org/)